Why Does Graphite Conduct Electricity: Real Reason
As we know graphite is made up of carbon and it is a very hard substance yet Why Does Graphite Conduct Electricity? Don’t worry in this article we are going to answer all the important questions related to graphite so without any further delay let’s jump right into the article.
Table of Contents
Why Does Graphite Conduct Electricity?
In simple words, Graphite can conduct electricity because it consists of free electrons in its structure which carry the electric current. Graphite consists of delocalized electrons which means each carbon atom present in it is bonded to three other carbon atoms, which leaves one electron free and this electron is responsible for the conduction of electricity.
By observing the below figure you can understand the structure of carbon:
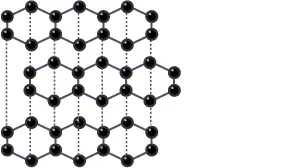
Structure and Bonding of Graphite
- Every carbon atom creates three covalent bonds with other carbon atoms.
- Every atom of carbon is arranged into layers with each layer consisting of hexagonal structural rings of atoms.
- No covalent bonds can be found between the layers.
- Each carbon atom consists of a single non-bonded/delocalized electron.
Properties and Uses of Graphite
Graphite is a crystalline form of carbon that has multiple properties and some of which are:
High electrical conductivity: As we already discussed earlier, graphite is a good conductor of electricity because of this delocalized electrons carry the electric current to other atoms. Due to this property, graphite is widely used in electrodes, batteries, and arc lamps.
High thermal conductivity: The real reason why graphite has a high thermal conductivity also concludes in its structure, the way the graphite layers are arranged makes it easier for them to slide away which helps in the thermal conductivity.
Low friction: Graphite consists of thin carbon atoms which slide on each other easily and it makes graphite a low-friction substance. Due to its low friction, it can be used in pencils as it is a very soft material and can be easily scratched off the lead. Graphite is also used as a lubricant in bearings and seals.
Chemical inertness: This is one of the most underrated properties of graphite, graphite has chemical inertness which means it does not react to most of the chemicals. This property makes graphite a good option where corrosion resistance is required like in nuclear reactors and chemical processing pieces of equipments.
Why Does Graphite Conduct Electricity but Diamond Doesn’t?
The answer to this question is pretty easy if you understood the structure of graphite, so in diamond all four carbon atoms are bonded to the other four carbon atoms which means there’s no electron to carry the current and that’s the reason “why does graphite conduct electricity but diamond doesn’t“.
To understand the answer deeply you need to understand the structure and bonding of Diamond.
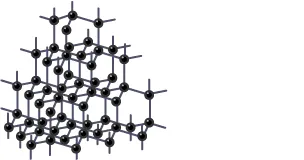
Structure and Bonding of Diamond
- Each carbon atom of carbon is joined to the other 4 carbon atoms by powerful covalent bonds.
- Rather than the hexagonal arrangement of atoms, carbon atoms form a regular tetrahedral network structure.
- No electron is free
Properties and Uses of Diamond
Hardness: Due to tightly packed atoms Diamond is considered the hardest naturally obtainable substance and features a Mohs hardness score of 10 which means it can only be shattered by another diamond. Diamond is widely used in cutting tools due to its hardness and heat conductivity, it can easily cut glass, metal, and other materials.
Transparency: Diamond is a transparent substance which means we can see through it. Diamond consists of extremely tight bonds in a tetrahedral structure that does not absorb visible light. Light is reflected and refracted many times before it exits a diamond which causes a sparkle of light in the diamond that we usually see. Diamonds are used as jewelry because it is rare, hard, and beautiful.
Heat conductivity: Strong covalent bonds are responsible for this virtue, strong bonds make it easier for heat to transfer quickly which makes a diamond a good heat conductor. Due to this it is used in lasers and cutting tools.
Chemical inertness: Just like graphite, diamond also features “Chemical Inertness” which basically means it will not react with most of the chemicals which makes it suitable for the making of diamond windows and diamond batteries.
Also Read: Why is Graphite Soft and Slippery?
What is Allotrope?
Allotrope is a term used to describe two or more different forms of the same element which exist in the same state. The division of allotrope can be derived from different types of bonding used in elements such as Carbon Allotropes. Carbon has three allotropes Diamond, Graphite, and fullerenes. Daimond uses Covalent bonds in a tetrahedral arrangement, Graphite uses Covalent bonds in layers/ van der Waals forces between layers while Fullerenes uses sp2 hybridization.
why can graphite conduct electricity?
Source: Chemistry by Desam Sudhakar AVC
Does Graphite Conduct Heat?
Yes, Graphite is a very good conductor of heat and electricity due to its structure and bonding. The graphene layers are stacked upon each other and due to weak bonds between layers, it’s easy for them to slide each other which makes it easier to conduct heat. The graphene layers are arranged in a way that makes it easier for the electron to travel and conduct heat and electricity.
Does Graphite Have a High Melting Point?
Yes, Graphite has a high melting point. It melts at around 3,600 degrees Celsius (6,800 degrees Fahrenheit). This happens due to the unique structure of a diamond, the atoms of carbon are tightly packed by strong covalent bonds, and a lot of energy is required to break them apart, which is why graphite has a high melting point.
Is Graphite Poisonous to Humans?
No, Graphite is not poisonous to humans. It is an allotrope of carbon and carbon is not toxic to humans however, it does not mean you should eat it for breakfast. Graphite can choke a human if consumed and also it can irritate the skin and eyes if rubbed onto them.
Which is Stronger Diamond or Graphite?
Diamond is stronger than graphite. Although, both belong to the same family, The elder brother (diamond) is incomparable to the little brother(graphite). Diamond is considered the hardest material in the world while graphite is used for its softness and slipperiness in pencils.
Conclusion
So there you have it, we have discussed everything from why does graphite conduct electricity to whether Is Graphite Poisonous to Humans. In the above section, you will find all the major properties of graphite and diamond, all major uses of graphite and diamond, and all the necessary questions related to this graphite and diamond. If you still have any doubts or queries you can ask them in the comments section.
